
A Taylor & Francis journal has retracted a widely-read paper linking cardiac-related mortality to COVID-19 vaccines after an unsuccessful legal attempt by the lead author to block the withdrawal. That author says he is considering further legal action against the publisher.
The article, “Risk of all-cause and cardiac-related mortality after vaccination against COVID-19: A meta-analysis of self-controlled case series studies,” drew swift criticism when it was published in Human Vaccines & Immunotherapeutics in August 2023. At the time, critics and sleuths were quick to challenge the data and methods used in the paper, which now has more than 143,000 views on the Taylor & Francis website and has been cited 15 times, including by two letters to the editor of the journal and a response from the authors, according to Clarivate’s Web of Science.
The retraction notice, posted online January 16, states the retraction resulted from concerns that arose about the methodology of the study and the integrity and availability of the data. The authors provided a full response to the queries; however, the publisher determined the validity of the findings remained in question, the notice states. It continues:
Continue reading Lawsuit fails to block retraction of paper claiming to link heart-related deaths to COVID-19 vaccines

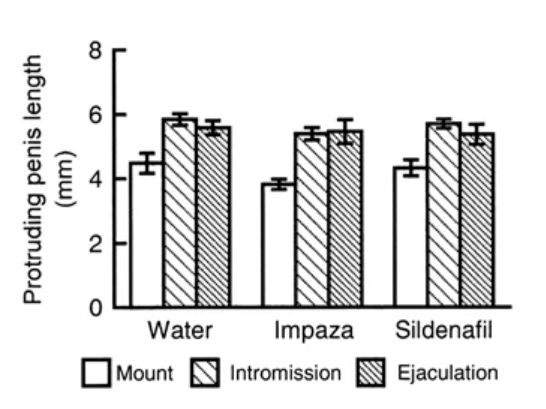

 For a host of reasons, a journal has retracted a paper co-authored by a researcher who reportedly once faced charges of practicing medicine without proper qualifications.
For a host of reasons, a journal has retracted a paper co-authored by a researcher who reportedly once faced charges of practicing medicine without proper qualifications. 
 When
When