PLOS One has retracted two papers from the Comet Research Group, a controversial cadre of researchers who, according to their webpage, seek “to find evidence about comet impacts and raise awareness about them before your city is next.”
The same research group was also behind a September 2021 paper — published in Scientific Reports and covered by Retraction Watch here, here, and here — that claimed a cosmic airburst flattened the city of Tall el-Hammam 3,600 years ago, providing physical support for the biblical story of Sodom and Gomorrah. The widely publicized paper was retracted last April after mounting concerns from outside researchers about the methodology, interpretations and data in the article.
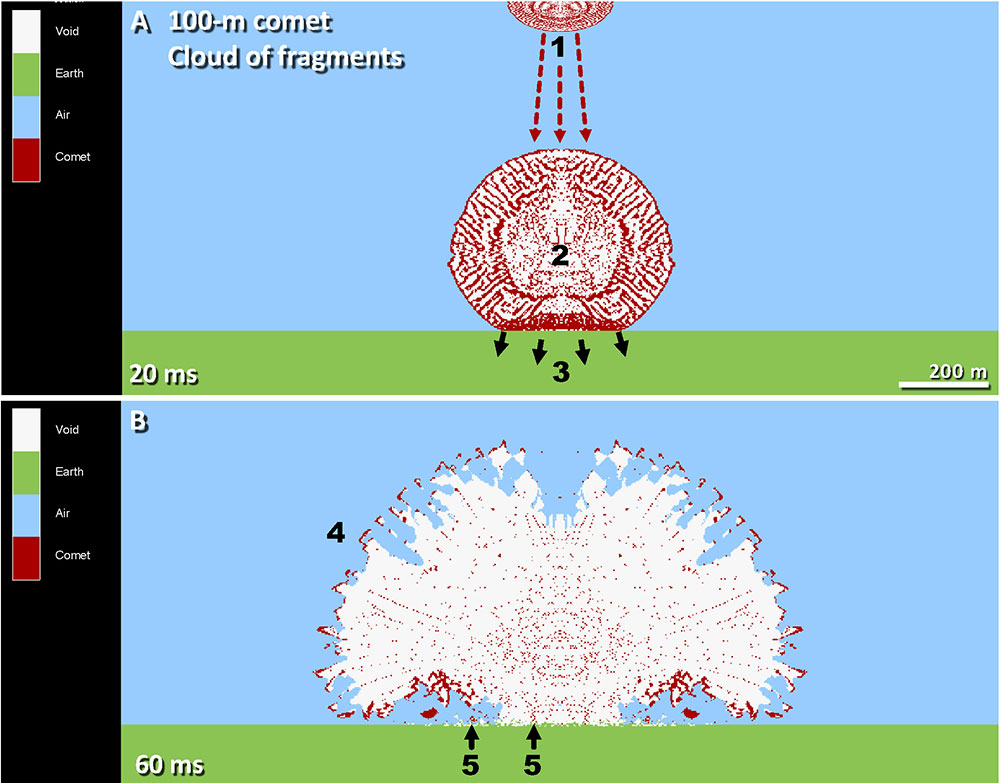
The group’s two new papers focus on different aspects of the Younger Dryas Impact Hypothesis, which posits that a “disentangling comet” broke up in Earth’s atmosphere before plummeting to the ground, initiating a comet-driven cataclysm that leveled humans and mammoths and much else approximately 12,800 years ago.
Continue reading Controversial comet theory struck by two new retractions