A study that a pharmaceutical company admitted last month included manipulated data will be retracted, Retraction Watch has learned.
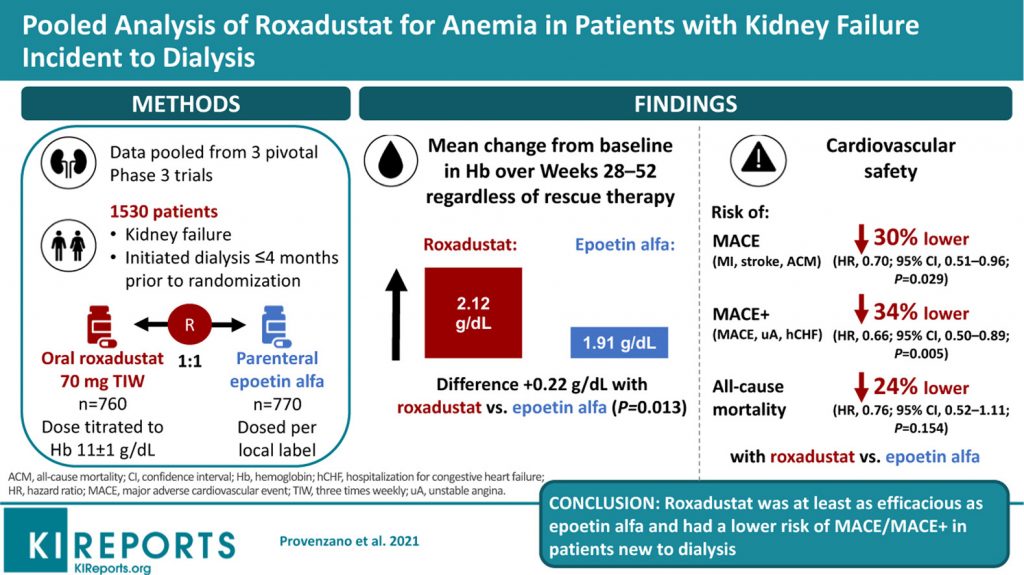
The paper, “Pooled Analysis of Roxadustat for Anemia in Patients With Kidney Failure Incident to Dialysis,” was published in Kidney International Reports in December 2020. The study analyzed data from a clinical trial for roxadustat, a drug intended to help anemic patients make more red blood cells. The medicine was tested in more than 1,500 patients with kidney failure that had been on dialysis for less than four months.
The paper compared roxadustat to a standard treatment, epoetin alfa. Epoetin alfa is not given to anemic patients who have kidney disease and are not dependent on dialysis, according to reporting in April by FiercePharma, because it can increase the risk of a cardiovascular event, including heart attacks.
In the study, roxadustat was as effective as epoetin alfa for these patients, but carried a 30 percent lower risk for death, heart attacks or strokes.
Then, on April 6th, Fibrogen announced, according to FiercePharma, that researchers had
changed parameters used to analyze heart safety data for roxadustat in patients with anemia from chronic kidney disease…The false criteria yielded more flattering data…
Those data, presented at a conference in 2019, suggested a good safety profile. But the re-analysis showed that patients on roxadustat actually had a higher risk of death, stroke, or heart attacks than the company had originally reported, and confidence intervals were widened.
Fibrogen said that they only found out about the blooper during a meeting:
“As members of senior management were preparing for the upcoming FDA Advisory Committee meeting, we became aware that the primary cardiovascular safety analyses included post-hoc changes to the stratification factors,” said Enrique Conterno, Chief Executive Officer, FibroGen. “While all of the analyses set forth below, including the differences in the stratification factors, were included in the NDA, we promptly decided to clarify this issue with the FDA and communicate with the scientific and investment communities.”
The company also said that they “continue to have confidence in roxadustat’s benefit risk profile.”
However, a spokesperson for Elsevier, the journal’s publisher, told Retraction Watch this week that the paper will be retracted and that the “authors will be given the opportunity to resubmit a corrected version.”
FiercePharma and a nephrology podcast, called Freely Filtered, reported on Fibrogen’s press release about the error back in April.
Fibrogen’s Kin-Hung Yu, the paper’s corresponding author, did not respond to requests for comment.
Like Retraction Watch? You can make a one-time tax-deductible contribution or a monthly tax-deductible donation to support our work, follow us on Twitter, like us on Facebook, add us to your RSS reader, or subscribe to our daily digest. If you find a retraction that’s not in our database, you can let us know here. For comments or feedback, email us at [email protected].
One would hope that Fibrogen transparently reports the data on safety of roxadustat, declares who is responsible for fudging the analysis, and that FDA demands this info for patient safety. It is unclear who manipulated the analysis to reduce the incidence of serious side effects of the drug, but Kin-Hung Yu retired (!) from Fibrogen late last year as in this link: (http://www.globenewswire.com/en/news-release/2020/12/01/2137424/33525/en/FibroGen-Announces-Retirement-of-K-Peony-Yu-M-D-and-Appointment-of-Mark-Eisner-M-D-M-P-H-as-Chief-Medical-Officer.html) .
If KHY is indeed responsible for the manipulated analysis, this would cast shadow on two other NEJM papers on the drug that are conducted in China, and KHY is the senior author on both (DOI: 10.1056/NEJMoa1813599 and DOI: 10.1056/NEJMoa1901713.)