The authors of a letter describing the synthesis of ketonitrones have retracted it, after new data showed that they incorrectly reported the product structures and the reaction mechanism.
We’re not sure what exactly went wrong with the original data in the letter, “Transition-Metal-Catalyzed Ring Expansion of Diazocarbonylated Cyclic N-Hydroxylamines: A New Approach to Cyclic Ketonitrones,” published in Organic Letters.
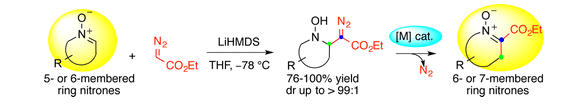
Here’s the reaction that the paper reported, from the abstract:
And here’s the very short note:
The authors retract this Letter because recently obtained X-ray diffraction data reveals that the structures of the products reported and the reaction mechanism described are incorrect.
The letter has been cited twice, according to Thomson Scientific’s Web of Knowledge.
We’ve reached out to last author Sandrine Py, who works at University Joseph Fourier in Grenoble, and to the editor in chief of the journal for more information. We will update this post with anything else we learn.
Like Retraction Watch? Consider making a tax-deductible contribution to support our growth. You can also follow us on Twitter, like us on Facebook, add us to your RSS reader, and sign up on our homepage for an email every time there’s a new post. Click here to review our Comments Policy.

It simply means that they should have waited to get the X-Ray data before publishing.
No, that’s not how it works in this field. You can rarely get a good structure of a small, organic compound mostly because you can’t get a crystal, never mind a big enough and unproblematic crystal, in the first place. The NMR evidence, if it’s reviewed by experts, is supposed to provide proof of connectivity. Also, follow up reactions, but those are almost never done. So, they must have made a mistake in the NMR characterization and the referees didn’t catch it. In this case yes, it would help to know what exactly the mistake was, but I can understand the desire not to tell us and just squash the paper.
Another retraction for a similar type misidentification of catalysis products a few months ago had an extra oxygen I believe… and that one is more unforgivable since a simple mass spec or elemental analysis could have told them that this is the case. In this case we don’t know. It could be just an isomer with the same molecular weight, and then it’s a lot harder when you do make a mistake, to identify it unless you have a good crystal. But requiring a crystal structure will not become the standard in the field. Probably ever.
Anyways, if I had to guess, take a look at Scheme 2, the carbene obviously forms with rhodium and it should give interesting chemistry. I would have expected they would have tried addition to something at this point, but it it goes by itself, it’s very likely not through forming intermediate B. If I had to guess, it’s probably CH insertion into one of the benzyls… but that would give a way different NMR. Maybe it’s still a ketonitrone, but the original five membered one, as that would give very similar NMR shifts, and the CH2 group is outside the ring. Which is useless since all you do is destroy your diazo moiety. There is enough catalyst and ligands for stray hydrogen to wreck the carbene. It’s a fun mystery at least, but very ‘academic’. I’m not going to waste a lot of time looking at their NMRs to solve it. Especially since they know the answer already.
To be precise (pedantic) the crystal structure(s) determined from the X-ray diffraction data obviously didn’t concur with their mechanistic proposals.
I think the fact that the products are different is more serious and is what actually matters. Mechanistic proposals in method development papers are pretty vague in general, at best. They are often not even wrong, or right. It’s the reaction and products that make the paper.