
Jacob Hanna of Israel’s Weizmann Institute has been a media darling for years, including as a member of the 2010 Technology Review 30 under 35 for his work with stem cells.
However, questions have been mounting about his research, both on PubPeer (which has critical comments for 15 papers he’s an author on) and in other stem cell labs, who have not been able to reproduce much of Hanna’s work.
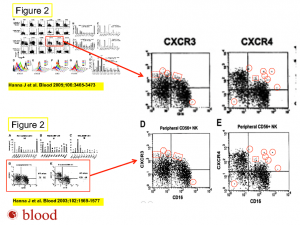
We asked Hanna about a PubPeer entry specific to a 2005 paper in Blood. Commenters have accused the authors of figure manipulation and possible data republication. Here’s a figure from that post:
On December 5, Hanna told us:
The Pubpeer comments have been brought to my attention earlier this week, and I am working with my former PhD advisor [Ofer Mandelboim] to correct the unfortunate and inadvertent mistakes in due course and ASAP.
Six of Hanna’s papers have been cited more than 500 times, according to Thomson Scientific’s Web of Knowledge. The most-cited, with more than 1,000 citations, was a 2008 Nature paper.
Many of the PubPeer comments on a 2010 paper in PNAS focus on other labs being unable to recreate the Hanna lab’s findings. According to September 2014 comments left on a Nature paper, supposedly by Hanna himself, an updated protocol for his method of manipulating stem cells is available on his website.
We’ve asked Hanna for further information regarding these allegations. Hopefully we’ll find out more soon.
Update, 2 p.m. Eastern, 12/17/14: Corrected name of Hanna’s PhD supervisor. Also corrected citation information; previous post only included papers with Hanna JH as author, but most of Hanna’s highly cited papers are Hanna J.
Another image (from Pubpeer) :
http://i.imgur.com/mnl9y6v.png
Hopefully he can rectify such things if they need to be… Many on Twitter are speculating that this is shaping up to be something quite bad.
This is a compilation of the allegations from PubPeer.
Retraction Watch readers can judge for themselves.
1) http://i.imgur.com/CWDQyx2.png
2) http://i.imgur.com/mnl9y6v.png
3) http://i.imgur.com/A1kBnnx.png
4) http://i.imgur.com/4udFLZW.png
5) http://i.imgur.com/V2vCLRp.png
6) http://i.imgur.com/sb86V8Q.png
7) http://i.imgur.com/zZeHu5n.png
8) http://i.imgur.com/eVJwTF3.png
9) http://i.imgur.com/ZMEiqRq.png
10) http://i.imgur.com/XU9DFs4.png
11) http://i.imgur.com/EASA0xn.png
I am not a member of the Weizmann institute, so it seems I can not access the protocol…
I think this may be it:
http://hannalabweb.weizmann.ac.il/wp-content/uploads/2014/10/Hanna-Lab-Robust-Reprogramming-of-human-Naive-iPSCs-form-Adult-Human-Primary-Fibroblasts-involving-Mbd3-Depletion.pdf
The links that RW gave to his website also seem to be broken. Should be:
http://hannalabweb.weizmann.ac.il/
[Editor’s note: Replaced the link, thanks. The original link wasn’t broken, but were somehow only available on the Weizmann campus.]
Dear Cat, I think you have a couple of inaccuracies here.
Jaenisch was not Hanna’s PhD advisor. Jaenisch is at MIT where Hanna did his stem cell postdoc. Indeed this is the lab where he became famous. But before that he was in Israel working on NK cells for his PhD. You will have to find a new supervisor. (Hint: check the 2005 Blood paper for clues).
Google Scholar suggests that Hanna’s most cited paper is the 2007 Science paper where he used a stem cell therapy to cure sickle cell anemic mice. Google says 1349 citations. Even allowing for the possibility that Google Scholar has a penchant to over count, surely the Cell review is not even close to being the top citation (384 according to the Goog).
Anyway, it is good you are keeping an eye on this story. It has all the ingredients of a classical greek tragedy. Other than not being set in Greece of course.
Jaenisch was not Jacob Hanna’s PhD adviser, but his post-doc adviser. The 2005 Blood paper is from Hanna’s PhD. This should be corrected in the post.
The Pubpeer comments have been brought to my attention earlier this week
Hanna’s claims to have only just heard of the problems with manipulated images are unconvincing when you read the defensive comments and the claims to priority in the PubPeer threads.
From the article: “On December 5, Hanna told us:”
Looking at the dates more carefully, I see that the earliest PubPeer query specifically addressing the images was November 30th:
https://pubpeer.com/publications/1319F6076BEA40C1BF6C22C96A4AEB#fb17520
— so that could fit in the “brought to my attention earlier this week” timeframe.
There are earlier comments in that thread, but mainly about irreproducibility, and whether researchers deserve priority for a cell manipulation even if the particular methods by which they claimed to achieve that manipulation have subsequently proved not to work.
“Many of the PubPeer comments on a 2010 paper in PNAS focus on other labs being unable to recreate the Hanna lab’s findings” Where have I heard this? Ah yes, STAP.
This may indeed be something very big and nasty, not only Hanna would be damaged, but also another blow for Nature and: for none other than the most powerful person in stem cell field, Jänisch himself. Imagine what is now going on behind the scenes.
He is Principle Investigator!
A paper published in Nature Genetics by a plant molecular biologist, Avraham A. Levy, also from the Weizmann Institute (Department of Plant and Environmental Sciences), is being questioned at PubPeer:
http://i.imgur.com/7wlAzc8.png
https://pubpeer.com/publications/021DF5B37988FD51AA82DF7EE91435#fb18961
Peer 0 states: “In 2007 I became aware of the problem raised in PubPeer and I immediately sent the originals to Nature Genetics together with a description of the changes that had been done explaining also that the obviously excessive treatment of the picture did not affect in anyway the validity of the results. The editor said they might post the original images as supplementary information in order to reassure readers of the authenticity of this data. For some reasons they did not share with me, they decided not to do anything.”
A few things here:
a) Hoe does Prof. Levy explain the odd banding in the figure and will he make the original gels public?
b) Did PubPeer already exist in 2007? If Prof. Levy was aware of the problems, then why did he not provide a response to PubPeer in 2007?
c) Who was the editor at Nature Genetics responsible for this case, and why did Nature Genetics not pursue a correction or erratum?
Perhaps it was poorly worded, but he probably means that there was a problem that was raised on PubPeer this year, and that he already knew of the problem and has known of it since 2007.
This is a very unfortunate situation. Hanna’s paper has dozens of co-authors and the criticisms related to 5 papers of his PhD. His advisor, Ofer Mandelboim, is the corresponding author of those papers and is the responsible to ensure the quality control of what comes out of his laboratory. Curiously, all readers/commenters appear to automatically blame Hanna. However, the main questions at this point should be directed to Mandelboim’s.
I suppose this all depends on the division of labour. I thought being the first author suggests that that one made the biggest contribution. So it seems fair to primarily address Hanna, at least in papers where he is the first author.
Jacob Hanna also just received the Kimmel Prize for innovative investigation at the recent board of governors meeting. http://www.weizmann.ac.il/acadaff/Kimmel.html This is an internal Weizmann award, but it gives about a million dollars for research so that you don’t have to waste your time writing grants for a while. It would be highly unfortunate for the Weizmann if the claims of misconduct were proven to be correct as that would probably mean Hanna cannot stay at his position. It would be an accounting nightmare; not to mention the BOG would all know about this case. At least Hanna, unlike some others, is active on PubPeer and is trying to answer his critics.
Jacob Hanna has made several breakthroughs in the stem cell field over the last 8 years. Simple inspection of these recent publications indicates his seminal contributions that are used by leading labs including Surani, Gribnau, Graf and others:
1) http://www.cell.com/cell/abstract/S0092-8674%2814%2901583-9
2) http://www.ncbi.nlm.nih.gov/pubmed/23533168
3) http://www.ncbi.nlm.nih.gov/pubmed/25640760
4) http://www.ncbi.nlm.nih.gov/pubmed/24336202
5) http://www.ncbi.nlm.nih.gov/pubmed/25569111
….
Jacob Hanna is currently, and by far, the most influential and best stem cell scientist in the iPS field, and almost the only one doing meaningful research. Every paper sets a new path.
A new paper just published in Nature by Marius Wernig (Stanford), independently confirmed the validity and authenticity of reprogramming obtained in Mbd3flox/- system.
http://www.nature.com/nature/journal/vaop/ncurrent/pdf/nature14274.pdf
Selected quote:
“We then wondered whether a similar intermediate population arises in high-efficiency reprogramming systems (12, 13). Published expression analysis of two high-efficiency systems showed transient CD73 upregulation, suggesting the presence of a similar intermediate (Extended Data Fig. 8a, b). We then characterized one of these systems, the Mbd3fl/− secondary MEF system, in greater detail (12). After confirming reported reprogramming efficiencies, we analysed this system by mass cytometry (Extended Data Figs 8, 9, 10). By day 3, fibroblast marker repression was evident, and CD73 was upregulated within this population (Extended Data Figs 8e and 9b). Within the CD73high/MEFSK4low population, CD49d (Itga4) upregulation was not apparent, but we noticed the emergence of a separate integrin, CD104 (Itgb4). By day 4, the major CD73high branch clearly overlapped with the CD104high branch and persisted into day 5. SSEA1high and CD326high expression was present on day 4, but clear co-expression was not seen until day 5. By day 9, CD73 and CD104 expression was dramatically reduced while CD326 and SSEA1 expression remained high. These data demonstrate a transient CD73high/CD104high population arises after donor cell program repression and before ESC marker acquisition, even in a highly efficient reprogramming system. Similar to CD49d and CD73, CD104 is not highly expressed in ESCs (Fig. 1a). And similar to viral reprogramming, adenosine treatment abolished reprogramming in the Mbd3 reprogramming system, albeit only at late stages, whereas compounds affecting CD49d function had little effect (Extended Data Fig. 4f).”
Correction published in Blood journal as indicated by Hanna.
http://www.bloodjournal.org/content/125/16/2583.2
Which was also questioned on PubPeer:
https://pubpeer.com/publications/C278F3DE939616C4ADBDB9C15DB268#fb28574
Another erratum.
http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(15)00165-4
All images supporting claims made about Hanna’s papers on PubPeer, and archived in imgur.com have been taken down. No explanation provided. This has taken place systematically in all these papers:
https://pubpeer.com/publications/8015433E22F7C41A03104E693B31BD
https://pubpeer.com/publications/20442331
https://pubpeer.com/publications/24048479
https://pubpeer.com/publications/24172903
https://pubpeer.com/publications/16037391
https://pubpeer.com/publications/18509334
https://pubpeer.com/publications/15578093
https://pubpeer.com/publications/19427283
https://pubpeer.com/publications/16892062
https://pubpeer.com/publications/15557145
https://pubpeer.com/publications/14604968
https://pubpeer.com/publications/12730110
The images that had been removed from PubPeer are assembled in one collage:
http://i.imgur.com/27vSfck.png
(available for how long?)